Charcoal syngas is approx 1/3 hydrogen and 2/3 carbon monoxide. This measure is for volume. One mole of CO is 28 g while one mole of H2 is 2 g. That’s a 28:1 ratio X 2 60:1? That’s a lot
That is only possible in a lab with a strict controlled system, fuel, environment etc. Nothing is eer linear ever on these systems; no matter how well its built and good you are. Your results will vary and widely.
I agree with MattR.
Far, far differences between desired, and mathed-out results versus real world results.
In mining what makes or breaks an operation is the difference between a low grade ore versus a higher percentage grade ore. 1-3% versus 7-12%. And the costs and expenses of separation then disposal of that “other” 88-99% of bulk not wanted, not needed.
This is why fossil coals and petroleums are such rich dense energy sources.
Then the flip side other side of the yeilds equations. You want the meat and fats as the high value out of a slaughter house operation. The blood, guts and offel?? Home , small operation then then just bury as soil enrichments. BIG operation then in accumulating amounts can be up-sold for values-adds too.
Just as the hundred years of literally worshiping dense rich carbon energies was short sighted; this current modern trend to worship hydrogen will be short sighted too.
The only way you can have some few being served up “cakes” (fancy high end restaurant fare) on a daily basis is if a whole larger majority accept eating daily plain wheat breads.
The very best of harvested wheats only produce a small percentage of cake grade of flour.
So never fall into the trap of being in your thinking; and lifestyles of being a dedicated, strident, insistent, loud noisy “cake-eater”.
Steve Unruh
-What about all the nitrogen that makes it through the system?
-Not all CO2 may be broken down. So it is still in the fuel stream
-Depends on exact composition of the charcoal- More lignin, better chance of hydrogen.
-Depends on humidity in the incoming air (H2O content and dampness of the charcoal.
-Depends on cracking temps in the reactor.
Am I missing something or getting something wrong?
I’m not sure what you’re getting at. I think the volume ratio is the molecular ratio. Isn’t all the chemical activity “by the molecule,” and therefore by the mole, and volume? The weight comes into play when you have to lift, move, or compress things.
Hello Kent. I don’t know whether you are responding to me (Pete) or to the OP. But, “by the molecule–”: means that you know exactly which molecule(s) you have in the input stream to the reactor and all the temperature zone effects that exist and are constantly chainging… That’s a big mess when it comes to “woodgas” in general.
----Just my thoughts…
Koen,
Thanks for the demonstration. What do you think is an effective, yet simple way to add water to the reaction of a charcoal gasifier to boost the hydrogen content of the gas?
The Co2 reduction is > than H2 increase
Convert it to steam and add as much as you possibly can that the machine can sustain. Im doing it partially in my chambered nozzled but I will be adding a preheat loop into the reactor to convert some water to steam while purging left over unconverted but preheated water into the nozzle.
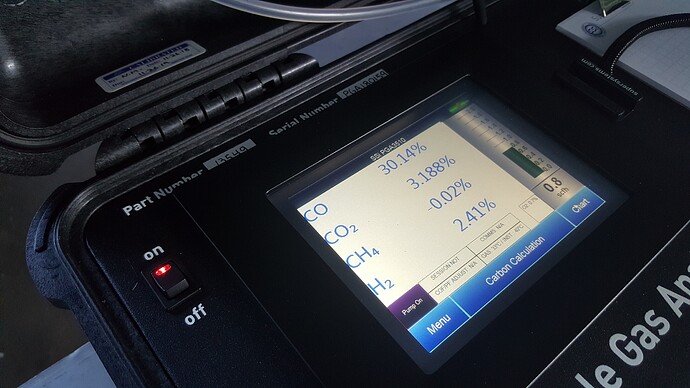
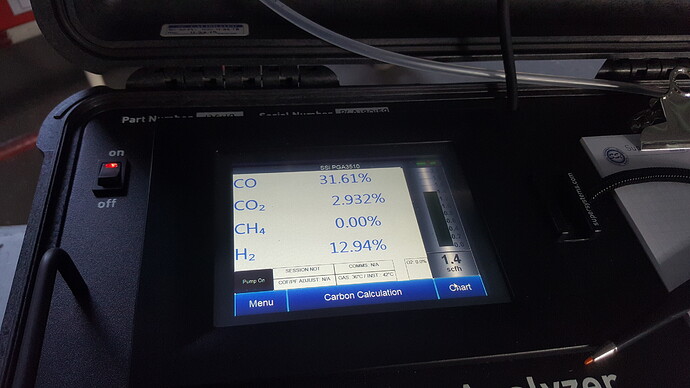
Greetings Koen Van Looken, thanks for the measurements, which I only dream of or imagine…
In Giorgi’s topic, we discussed a charcoal gasifier and theoretically I came to the conclusion that in practice this gasifier requires about 1.5 kg of air and about 0.6 - 1 kg of water vapor per 1 kg of charcoal. This means that in this system it is very good to preheat the air and steam with the engine’s waste heat, this way the gas quality increases significantly, since we do not burn so much charcoal to maintain a high temperature, and at the same time the proportion of air decreases, and the proportion of water vapor can be increased.
Here it is interesting to compare a charcoal gasifier with a wood gasifier. Wood contains carbon, oxygen and hydrogen, so there is no need to add water vapor, since there is already too much of it and it must be eliminated from the process. I assume that the wood gasifier also needs much less fresh air to operate, probably 3x less (approx. 0.5 kg per 1 kg of wood), which means that this air can be effectively preheated with hot gas. The upper part of the gasifier - the condensation zone - should be cooled to 60-80 °C, this would ensure optimal humidity of the pyrolysis gases, but lower down before entering the hot zone it would be good to heat these moist gases as much as possible, I am thinking of using the engine exhaust gases for this.
Less air also means less nitrogen in the gas and that is good.
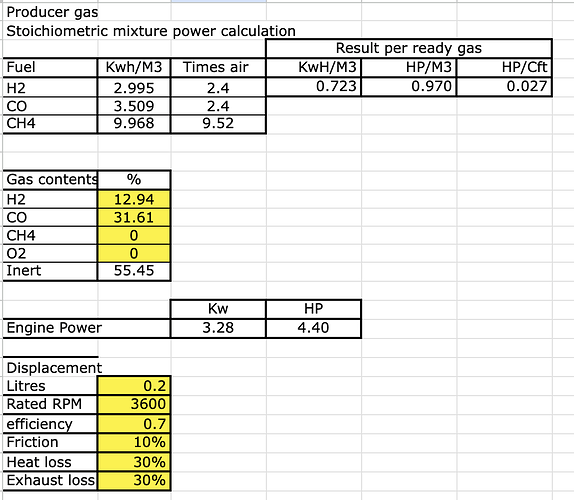
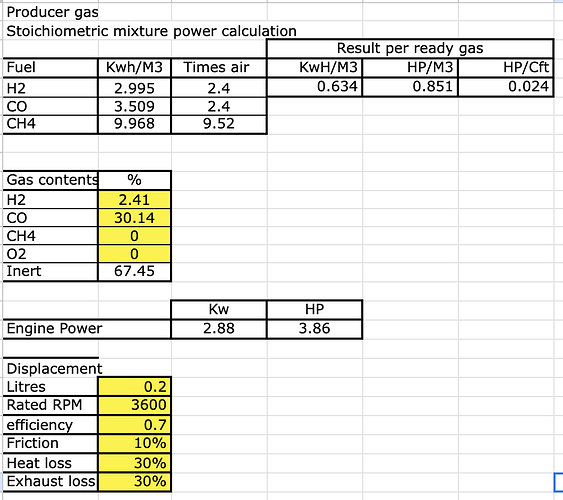
Calculations based on the previous pictures, with an engine size of 200cc, running at rated 3600 rpm.
You can see how much more HP it can make
from 3.86 Hp to 4.4 Hp ( = +14%)
second advantage, if the engine ignition is not advanced then probably you don’t reach 3600 rpm without the added water/hydrogen.
third advantage, less clinker formation as the added water does reduce the temperature in the reactor.
In answer to how much water and how is the best way: the more you can use waste heat from the wall of your gasifier , the more you can add, where as your reactor temperature is key reference, keep it orange/yellow glowing, not run it dark/dull red.
If your engine ignition is advanced, then the 3% CO2 does more or less prevent knocking.
Pete, you’re surely right about woodgas being complicated. I was puzzled why the OP thought CO being much higher in mass than H2 was surprising or important. It seems to me stoichiometry is based on counting molecules, and volume is proportional to molecules. Mass may vary a lot, but doesn’t effect things like mixing very much.
I’m puzzled by lots of things, but life goes happily on, just the same ![]()
koen, thank you very much for the documentation…have you seen - noticed - different flame colour in the flare with more or less h2 content?
i have observed with fresh coal a orange flame, when the coal was reused from the last run in the gasifier, than the flame was blu, without any traces of orange…than adding water drops in the nozzle, the flare becomes orange again…
Tone - you make a good point about wood gas and its components and how it wants heat.
Charcoal generates a surplus of heat and so benefits from water drip. The water absorbs excess heat via water-shift, upgrades the producer gas and moderates temperatures. No tars so no worries about the reaction getting a little cool.
Wood is challenged to get enough heat to take all the chemistry to completion (and crack tars). Tone, while you say wood needs less air, I think wood needs so much more heat that additional combustion is necessary, so more air than chemistry suggests. Preheating that air makes a lot of sense. Exhaust gas temperatures are quite high and would serve to bring combustion air to very high temperatures if you use counterflow techniques.
Any heat carried by the combustion air is heat that doesn’t need to be created through combustion. That’s a sure plus.
Some very enlightening explanations here now.
But back to the topic originators question of how much hydrogen is really in bio-mass derived(?) synthetically made fuel(?) gas.
The first, most important guiding criteria would be what is your purpose for the hydrogen??
Many of the responses are from respondents who’s usages are for their Internal Combustion engines fuel blends.
Far, far different than those arm-chairing; white-boarding for a pure hydrogen feed-stock. A feed-stock to use as a pure input for fuel cells; as a buoyant gas; or a recombinant long-chain builder. All other elements then being just annoying “waste” by-products.
Well here-is-the-rub; here is the real world reality . . . all factors do count, and will assert themselves whether you account for them or not.
All bio-masses; all petroleums; all fossil coals will have percentages of minerals and metallics in them.
Forced rapid thermal decompose any of these and you will have these then exposed freed up minerals asserting themselves.
My fir and spruce woods are some of the lowest mineral-ash at only 1%.
Consume up a few hundred-weight of my wood and that 1% accumulates requiring removal to allow for further desirable processing:
Lower temperature fluffy ash having all of the hydrogen and carbons released; used up removed.
Then center of the fire-box; forced combustion higher temperature fused clinkers:
As I said in just three day and three hundred-weights of wood consumed I willl have 3-5 pounds of mineral-ash produced:
A waste? Hell NO. Full of minerals concentrates for the plants growing soils.
Composite Woodgas and Chargas inside an internal combustion engine and all factors can be desirable except for mineral ash and excessive moisture.
As was found in the late 1960’s, and KVL pointed out a percentage of inert CO2 in the blend can be a desirable TP spiking dampener inside of an IC engine.
And an internal combustion cycling engine benefits a lot from air-intake in atmospheric nitrogen and argon as heat boosted expansion gases. And these do not lubricants cylinder walls wash, then wear like steam does.
Look back up at KVL’s numbers . . . the CH4 (made methane fuel gas) 3X plus the energy value as H2 hydrogen. The made CH4 has again just shy of 3X the energy value as CO fuel gas.
One of the many reasons I will continual insist on woodgasing versus a charcoal gasing. Raw woodgasing makes CH4. Charcoal gasing does not.
And since I will make only for my own in-engines usages then it is me, myself, who like others driving daily; can balancing act juggle for benefits all of the factors.
When you make for others to easy-use consume; then the delivered must be dumbed-down extremely simplified for their minimal thinking consummations.
Steve Unruh
Whoa. Is that biomass or charcoal? Thank you
Thank you that’s a good reply
Hi Phillip,
This is a pure charcoal gasifier