You may have heard that boiler’s mates, in the Navy, will wave a broomstick in front of themselves when they go into a compartment where they suspect a steam leak. If the super heated steam cuts a piece off the broomstick they know approximately where the leak is. The super heated steam actually gasifies the wooden broomstick.
Steam can also gasify charcoal to produce water gas. So, I wonder, has this been done on board a vehicle?
Rindert
H2O + C → CO + H2. By 1970, however, relatively little hydrogen was being produced by such processes. Water-gas A mixture of carbon monoxide (CO) and hydrogen (H2) produced by passing steam over red-hot coke using the endothermic reaction C + H2O # CO + H2
Never heared about the Navy anecdote, but l researched quite some about water gas.
What you drawn wuld absolutely work. One problem thugh… l done some math before here but cant find it. Anyway, for this to work, steam shuld be heated to insane temperatures, we talk a couple of thousant degrees. The reaction is extremely endothermic.
Yes, the reaction is extremely endothermic.
I think maybe 1100C will be required. This is not insane.
We will have to use about 1 pound of water for each pound of charcoal. I have to do an energy calculation to see how powerful the steam maker should be.
Rindert
Could be meaningful
CO + OH → CO2 + H
Henry,
I prefer C + 2OH → 2CO + 2H
Same process, different temps, different pressures, different dwell times…
[
](Profile - k_vanlooken - Drive On Wood!)k_vanlooken
I think historically there have been more injures , causalities from steam then wood gasification . Historically there have been more injures , causalities from timber industry than any other occupation .
As this pound of water boils at 212°F, heat energy is being added to change it to steam. When the last of the pound of water has been vaporized to steam, the stove will have added 970 BTU’s to that pound of water to furnish the energy to make what we call a pound of steam.
[image]
ir.library.oregonstate.edu › d…
ALL YOU’VE EVER NEEDED TO KNOW ABOUT STEAM BUT WERE AFRAID TO ASK
And this is just to turn it to steam not any hotter then to vaporize it.
Bob
Isnt this what we are already doing with our water drip and steam injection systems?
Im just pouring a stream in my machines and it is cracking just fine. There is no moisture dropping so that water has to be converting. (my assumption)
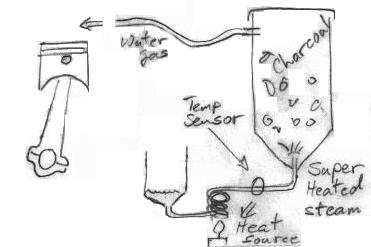
My next gen reactor, I am injecting water at the throat of the hearth to flash steam and super heat it. It should be crazy hot here well above 2000*F. The steam then migrates up and into the air intake of the unit leading into the hearth.
No, not exactly. Water gas doesn’t contain any nitrogen, carbon dioxide or other inert gasses. So the engine should make more power.
And, potentially, a very simple to operate system. A, proverbial, ‘little old lady’ might be able to operate it.
Rindert
So the steam itself is providing the heat for the process I take it. Seems that would be difficult but an opportunity to think outside the box here. Combine the retort/kiln process into the heat generation for the steam while it produces the charcoal. Charcoal consumption I could see going way down while you crack more water. The less charcoal required the more viable this system.
Yes yes I do see charcoal technology advancing with higher volumes of water cracking. That is exactly my mission.
Errr. Sanity check Matt. There is so much energy in a pound of charcoal an no more. The engine might make more power because it’s fuel contains less inert gas. Okay. But all the energy to operate the whole vehicle must ultimately come from from the charcoal.
I suspect you will get slightly less fuel economy, but I’m pretty certain you are not going to get more.
If an electric heater is used to make the steam then we may need a higher output alternator.
You lost me, are you not factoring in the energy from the water??
I ltr of water is 15,000 BTU added. Ive cracked 90,000 btu with my simple water drip using the super charger in one hour. Cut my hopper consumption nearly in half. That’s not factoring in the Oxygen conversion but that Oxygen is providing some oxidation of the carbon so you are probably getting little CO from the O to carbon shift.
And yes the gas energy density will go up besides the added H2 as the inert gases are no longer.
This is why I want to play with pure oxygen or a concentrator to help reduce the Nitrogen in the gas and possibly boost the temps to get more water into the unit.
If there is no nitrogen in the mix, what is going to happen in the firetube reactor? How can we build this with out melting the metals down? It would be generating heat up into the 4000+ degrees Fahrenheit.
Have you ever used or built a HHO generator with a torch? Mine has finally gone bad and I need to rebuild it. It is some very explosive gas. Especially if it get some air added to it at the wrong time.
Let’s say we double the burn speed of the wood gas and I am speaking on this being used in a vehicle gasifer not stationary. Would it still be safe to use. Just thinking of safety here. Just getting a buff back on the hopper lid when opening it might be a boom instead.
The safe way is to leave the wood gas alone and make your compression a little higher on your ICE and run your vehicle on premium fuel. Like Joni doing, but he increased his compression much higher and runs only on wood gas.
It is okay to make the gases rich but not dangerous by to much Hydrogen in the mix if that is possible. Think of it this way the Nitrogen keeps things stable in the wood gases.
A great test would for you Matt to try it in a safe way on oxygen with out nitrogen and see how stable it is if you add extra air with 800 degrees Fahrenheit wood gas in a safe laboratory experiment setting. I have messed with hydrogen/oxygen/nitrogen mix very explosive and fast burning and hot with my HHO generator.
Bob
HHO is how I got here. lol H2 is the gas we are after, regardless of the amount of nitrogen the gas is dangerous if oxygen is in the mix and is why we have safety valves. So Im not worried about flash backs and yes I know how powerful and quick HHO is.
Once the gas leaves my gasifier it is never in any large vessel to accumulate and it is also under vacuum so it is low density in the hose and pancake filter. the hose will pop off if there is a flash back and there is not enough gas in there at any given time to be dangerous. The pancake filters are very low volume devices.
More extreme heat is the whole point, I want this to be unable to operate with out water. That is the whole point to make this crack water for power and charcoal is only the catalyst for the process. If it melts down without water then I succeeded.
But you see the point is to reduce the atmospheric air while maintaining the amount of oxygen in process… As the steam expands in the process it chokes off the atmospheric air so you are limited. With pure oxygen it will be denser and allow me to expand more steam in the process.
Yeah thats what I am doing with the super charger, its adding compression ( I think no boost gauge yet) . It is also making the gas flows more stable. I think that is where Im getting the advantage and not so much the boost. I need to get a gauge to see if Im even boosting the engines yet. The generator doesnt seem to care if its powered on our not. But after I have this running and turn it off after a bit I do loose quite a bit of power. Im only just starting and its way to cold for my bones to be fiddling around with a gasifier outside. Ill pick back up on this soon. I have a revised super charger Im building now.
Note the line to the explosion, that is the gas feed tube to the what ever it was I was blowing up here. That was a 60 FPS camera I took this still out of. Generally you get about 2 to 3 frame window. Ive blown lots of stuff up with HHO about all its good for. lol


The steam expansion, I believe is also why we see such a boost when we are adding our water drip systems. Its not just the added H2 I think it is also the reduction of atmosphere entering the systems as the steam is expanding and displacing it. Direct water feed into the hell hole I think have an advantage over flashing to steam first. When that water hits the hell hole it dont stand a chance as long as you are not over doing it. Its less displacement of the needed air for oxidation.
Nice pick matt ,i blew up quit a few pop cans, and a few small plastic bags,with hho, before i found drive on wood .com, my ears were still ringing from the fast burn bang.
Rindert, unfortunaly its more close to 4000c (7000f)… if l remember right. Keep in mind the reaction only works over 800c and fast enaugh over 1000.
Cmercialy this huge energy demand was achived by haveing 2 gasifiers. First, superheat the carbon in one by injecting air in. Then, close the air and inject pure steam. The 2 gasifiers alternated between the working and regeneration cycles. Wastefull, bulky but its the only way possible at the time.
Bob, safety in a engine is not a problem. Keep in mind that first of all the gas contains 50% of slugish CO not just pure H2. Second, since the gas is richer, there is much more air mixed for a percect mix (not 1:1 like woodgas but more like 1:3 from the top of my head) and that air carrys its own nitrogen in the mix.
I am sure you even driven on such a gas before  imagine pushing the truck to the max for miles. Then suddenly stop. The extreme heat will continue to make nitrogen free “superwood gas” that fills your system. Unless you adjust your air mix, the engine will stall at takeoff from extreme richness. But if you do adjust the mix in time, for a few seconds you have power compareable to gasoline.
imagine pushing the truck to the max for miles. Then suddenly stop. The extreme heat will continue to make nitrogen free “superwood gas” that fills your system. Unless you adjust your air mix, the engine will stall at takeoff from extreme richness. But if you do adjust the mix in time, for a few seconds you have power compareable to gasoline.
To summerise, l think the idea to efficiantly drive on actual nitrogen free watergas is unrealistic. But its not black and white! Who says we can only have 50% or zero % nitrogen in our gas? We can just partialy heat the steam (clue: hopper gases are mainly steam  ) to the best of practical abilitys and REDUCE nitrogen rather thain iliminate. Like l sayd so many times, every calorie put in the gasifier makes a calorie richer gas…
) to the best of practical abilitys and REDUCE nitrogen rather thain iliminate. Like l sayd so many times, every calorie put in the gasifier makes a calorie richer gas…
I can not work through the simple stuff . Now I am going to add regenerative braking to a wood gas vehicle . When you apply brakes braking power thru drive-train pumps wood gas into accumulator . when you drive away this gas is proportioned out .
https://www.wardsauto.com/news-analysis/f-350-tonka-hydraulic-regenerative-braking
Hmmm, This does not seem right to me, because the reaction zone never gets that hot, and we are already ‘cracking’ some water there.
I looked up the energy content of steam at .1MPa (1 atmosphere), and 1100C; 4257KJ/Kg. From chemistry I make a rough estimate that about 1 pound of water will be used for each pound of charcoal. 3.5mi/lb comes from information on this forum for a small car. I get 10.7KW or 14.4 horsepower to make the superheated steam. This could be completely wrong, but at this point it seems about right to me.
Rindert
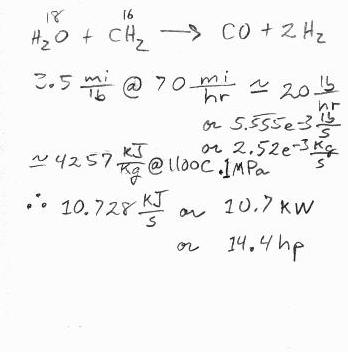
It certainly reduces airflow through the nozzles. And this would reduce air drag. But what I propose would eliminate air entering the gasifier at all. The suction from the engine would draw liquid water into the steam generator where it would be heated to 1100C. Obviously the water is going to expand enormously, but it will remain at the same pressure we usually see in gasifiers. The amount of vacuum on the system will determine the flow rate of water into the steam generator.
Rindert
Thank you Kristijan, YES, I have experienced this when driving my truck. And have really notice it when I have made my mix of charcoal and wood combination for fuel. I thought it was my mix I made that was doing this and really making good gas at the moment. But it was the gasifer for a moment because of the extra stored up heat making H2O extra oxygen with out the Nitrogen in it. It was really noticeable when my hopper was getting low on fuel and I had my rocket fuel mix in the hopper. I think using dry wood with a little moisture in the charcoal to control the black dust can help by a higher volume of the H2O in the charcoal. It is like a sponge with miles of voids in every piece of charcoal and is a filter with H2O in it. Burning it might be causing this super extra oxygen effect with in the charcoal itself. So it is not all just about Hydrogen being made from raw wood to charcoal, it is also Oxygen being made being used with out the extra Nitrogen accompanying the Oxygen. And it is being done in side the white hot burning charcoal super heating and then strip off the Oxygen leaving only Hydrogen. Okay The Light Bulb Just came on in my head. Now I see what you guys are talking about. In a wood gasification by using moisture in the charcoal with the wood it helps to increase this H2O to H H O separation affect. All it takes is more heat.
There is however still Nitrogen coming in with the expanded hot preheated atmosphere air from the gasifer preheater and engine preheater.
Questions: does Nitrogen being a inert gas get displace by other gases if heated to high temperures because of the different gas properties in their structure of how they are put together and expand? I would think yes, but I do not know for sure. We know some gases are more stable then others, like Nitrogen gas is more stable then Hydrogen gas. So can we use this for our add vantage some how? Or are we already doing this?
I am not a chemist, so I do not know.
Bob
